elisa test quality control|elisa testing standardization : convenience store The enzyme-linked immunosorbent assay (ELISA) is a commonly used analytical immunochemistry assay based on the specific bond between the antigen and the antibody. The application of this test has significantly changed the practice of medical laboratories in which it is used for detection and quantification of molecules such as hormones, peptides, antibodies, . Master the word "AUTOCLAVE" in English: definitions, translations, synonyms, pronunciations, examples, and grammar insights - all in one complete resource.The principle behind an autoclave centers on the use of moist heat to achieve effective sterilization. This process involves steam at high pressure to elevate the boiling point .
{plog:ftitle_list}
If space is limited in your steri-center, the M9 makes a good fit. This sterilizer is designed to .
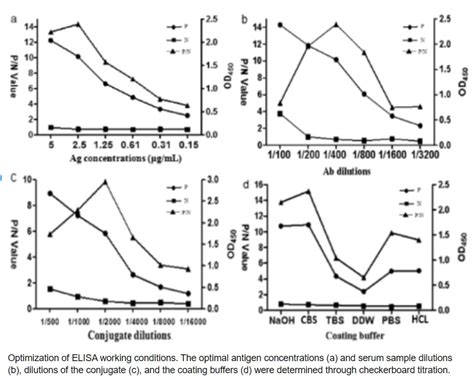
Evaluation of specificity may be conducted during optimization and validation, .High flexibility and sensitivity: both direct and indirect methods can be used. Demanding . The enzyme-linked immunosorbent assay (ELISA) is a commonly used analytical immunochemistry assay based on the specific bond between the antigen and the antibody. The application of this test has significantly changed the practice of medical laboratories in which it is used for detection and quantification of molecules such as hormones, peptides, antibodies, .Background: Internal quality control (IQC) samples may be incorporated in enzyme-linked immunosorbent assay (ELISA) routinely for detection of errors occurring due to change in environmental conditions, test system, or operator performance. We have described methodology for preparation of IQC samples, monitoring of results using Levey-Jennings (LJ) charts and .
Herein, we seek to develop such a set of quality control tools. These tools are tested using the development of an ELISA to detect a novel monoclonal anti-cocaine Fab fragment that is in preclinical development for the treatment of cocaine overdose (Kirley and Norman 2015).This provides an excellent test dataset, as it represents an example of a drug . ELISA is an antigen-antibody reaction technique used to detect substances like proteins, peptides, antibodies, and hormones. There are three main types of ELISA - indirect ELISA detects antibodies, sandwich ELISA detects antigens using two antibodies that bind to different sites, and competitive ELISA measures antigen concentration based on competition .
The ELISA is a rapid test used for detecting and quantifying antibodies or antigens against viruses, bacteria, and other materials. This method can be used to detect . quality-control program must include a quantitative account of these effects. It is beneficial to have a method in place that allows you to perform regular, A competitive ELISA, also known as an inhibition ELISA or blocking ELISA, is possibly the most complex of the ELISA techniques. Originally developed in 1976 7 for the detection of human choriogonadotropin, the assay works by detecting interference to an expected output signal level, producing an inverse relationship.
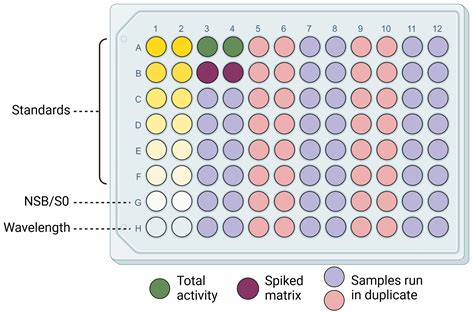
Enzyme immunoassays (EIAs) use the catalytic properties of enzymes to detect and quantify immunologic reactions. Enzyme-linked immunosorbent assay (ELISA) is a heterogeneous EIA technique used in clinical analyses.[1] In this type of assay, one of the reaction components is nonspecifically adsorbed or covalently bound to the surface of a solid .Here we explain the various types of control samples you should use when running an ELISA. Positive control. Use either an endogenous soluble sample known to contain the protein you are detecting or a purified protein or peptide known to contain the immunogen sequence for the antibody you are using. A positive result from the positive control . The application of immunoassay techniques contributes tremendously to the quality control and safety of our food supply. . were added to presumptive positive test samples resulting in a negative .
To evaluate the accuracy of the sandwich ELISA method, three quality-control samples with concentrations of 40, 10, and 2 μ g/mL were used, and the recovery values were 104.01%, 102.22%, and 96.13%, respectively (Figure 3 A), indicating the good accuracy of the ELISA method. The reproducibility of the sandwich ELISA method was assessed with .ii The Department of Immunization, Vaccines and Biologicals thanks the donors whose unspecified financial support has made the production of this document possible. 2.1 General Technique of ELISA 2.1.1 Principle. ELISA presents antigen–antibody reaction. ELISA was introduced by Peter Perlmann and Eva Engvall in 1971 at the University of Stockholm, Sweden [].This is a popular laboratory technique used to test antibody or antigen concentrations in different samples (Fig. 5.1).ELISA is a plate-based method, where an .
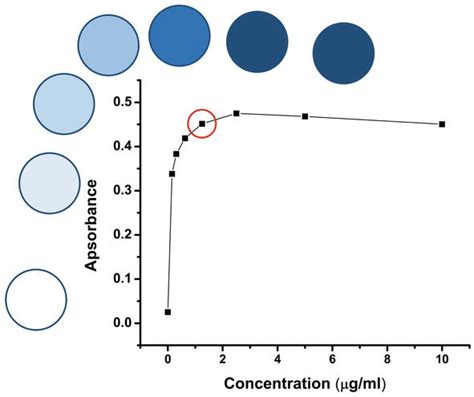
The test is an indirect competitive ELISA for the congener-independent detection of Microcystins and Nodularins. It is based on the recognition of Microcystins, Nodularins, and their congeners by specific . 0.75 ± 0.185 ppb, 1.5 mL, prepared from a secondary source, for use as a Quality Control Standard (QCS) 4. Sample Diluent, 25 mL, for . Figure 2. Standard curves of a Sandwich (left) and Competitive ELISA (right). Endogenous Protein Controls are only used during development of ELISAs for protein targets. If recombinant proteins are being used as standards, it might be necessary to confirm that binding and detection of endogenous protein by the antibody components of the assay follows the .
Quality control products encompass Fixed Quality Control(F-QC) and Random Quality Control(R-QC). F-QC limited in clinical applicability, cannot fully represent actual specimens or handle unexpected scenarios such as instrument or reagent failures [11].In ELISA testing, its shortcomings primarily manifest in its inability to monitor every well on the ELISA .
Three ELISA's for the detection of staphylococcal enterotoxin A (SEA) were evaluated by a collaborative test in five laboratories for possible use in quality control laboratories. Two ELISA's gave .However, the principles of the ELISA remain the same. There has been some rearrangement of chapters plus addition of three new ones dealing with charting methods for assessing the indirect ELISA, ruggedness and robustness of tests-aspects of kit use and validation, and internal quality control and external quality management of data, respectively.We evaluated measurement uncertainty and performed internal quality control of an ELISA test for allergens in egg and milk, using control samples. For the evaluation of measurement uncertainty, the following three important factors were identified: 1) Differences in test-kit lots, 2) Different-day reproducibility, 3) Same-time reproducibility. .
negative elisa controls
Quality control for qualitative assays: quantitative QC procedure designed to assure analytical quality required for an ELISA of hepatitis B surface antigen . The assay protocol for Ortho Antibody to HBsAg ELISA Test System 3 specifies running 3 negative calibrators and uses a test of their range, as well as a test of their mean absorbance .
Vaccine potency is used as the most crucial index of quality control, and the National Institutes of Health (NIH) test is the traditional gold standard for assessing potency [4]. . In vitro ELISA test to evaluate rabies vaccine potency. J Vis Exp (2020), 10.3791/59641. APPLICATIONS OF ELISA 1- Hormones 7- Vaccine Quality Control 2- Proteins 8- FOR GMO (Genetically modified organism) 3- Infectious Agent ( Viral, Bacterial, 9- For Rapid Test Parasitic, Fungal ) 4- Drug Markers 10- IgG, IgM, IgA 5- Tumor Markers 11- In New Born Screening 6- Serum Proteins 12- In Clinical ResearchThe statistical side of ELISA (the mathematical form of the standard curve, curve fitting method, and concentration estimation method) is not affected by these quality control measures intended to reduce operational uncertainty; Qian et al. showed the estimation uncertainty due to statistical reasons is considerable. As such, this is where we .
Data from standardized internal quality control (IQC) samples were used to assess ELISA performance indicators with reference to expected upper and lower control limits, as determined from studies by the kit producer (tentative values). . assay proficiency with respect to the accuracy of assessing whether the IQC samples tested positive or .
The use of semi-quantitative assays such as the enzyme-linked immunosorbent assay (ELISA) requires stringent quality control of the data. However, such quality control is often lacking in academic settings due to unavailability of software and knowledge. Therefore, our aim was to develop methods to . Because ELISA is the most commonly used immunological test method in clinical practice, the ELISA test is used as an example to introduce related issues. The Quality Control Laboratory of the Clinical Laboratory Center of the Ministry of Health began the nationwide evaluation of the quality of hepatitis B marker tests since 1988.Quality Assurance Upon receipt of a customer order, we assemble and test each kit to ensure that it meets specifications prior to shipment. The kit assay range, sensitivity and precision, as well as the final parameters for each specific kit are confirmed with the customer. Porcine circovirus type 2 (PCV2) is a highly prevalent virus in pig farms worldwide that causes significant economic losses in the swine industry. The PCV2 virus-like particles (VLPs) are potent subunit vaccines that are widely used. Currently, the adopted quality control of VLPs vaccines is mainly based in animal testing, the titration of neutralizing antibodies, or .
elisa validation definition
The forceps are for single use only and are provided sterile, and are nonpyrogenic in the patient contacting area from the tip to the bend in the forceps.
elisa test quality control|elisa testing standardization